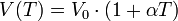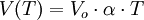Leaves are lateral appendages of the trunk and its branches. They are often green in them because they contain a lot of chlorophyll, a pigment needed by plants for photosynthesis, which allows them to "breathe." The leaves are also the site of gas exchange with the external environment.
There are many types of leaves, even if we know the most nomofilli , that the leaves on the branches are seen!
But there are also embriofilli (cotyledons) that are inside the seeds (if only monocots, dicots if there are two), the catafilli found in barrels in the ground, the ipsofilli (bratee) of flowers, the whole fruit (sepals and petals of flowers), the sporofilli (leaves carrying spores, which are necessary for reproduction).
leaf as it classical, is characterized by a sheet, a petiole and stipules, which are short appendages at the base of the stalk. The foil is the green (or color) of the leaf. Some leaves are even colorless ( plant Aucuba ) or stained for the presence of red pigments called anthocyanins. When the leaf is about to fall to the ground becomes of other colors such as yellow, orange or red, as it moves from leaf chlorophyll, and this is filled with carotenoids. The stem is that part that starts from the branch and arrives at the base of the lamina, a dark color.
The leaves possess ribs, which are those streaks that you see with the naked eye and feel by touching the plate (especially at the bottom of it). Depending on the types of ribs, there is a classification of the leaves:
1) uninervie leaves (only one rib, such as conifers, pine for example);
2) parallelinervie leaves (veins almost parallel to each other, such as maize);
3) penninervie leaves (a the leaf midrib divides into two almost equal parts, the common characteristic of the leaves, such as the apple);
4) palminervie leaves (the leaf is shaped like the palm of your hand and slide the ribs in each "finger ", as the screw);
5) leaves peltinervie (ribs start from the center of the leaf to go in all directions, like spokes of a wheel).
There are also free leaf veins, such as those of Gingko Biloba , leaves very old and unique in the world, with grooves parallel to each other and particular shape.
Other classifications grouped in pinnate leaves (a pen), palmate (shaped like the palm of your hand), and peltate.
The deciduous are those who fall each end of the growing season (every season), while the persistent leaves are evergreen plants and last longer than a season.
Each leaf may also have margins of , which are divided into whole, serrated, toothed, crenate, wavy, lobed, parties.
apple leaf

grape leaf