Gay Lussac was a French-born scientist, who discovered that when two gases react, the relationship between the volumes of reagents and products are always the same as the relationship between whole numbers.
For example, a volume of hydrogen reacts with chlorine giving a volume of two volumes of hydrochloric acid, according to the reaction:
H + Cl → HCl
Again, two volumes of hydrogen react with oxygen to give a volume of two volumes of water, according to the reaction:
H + O2 → H2O
First Law of Gay Lussac : constant pressure, the volume of a gas increases with ' increasing temperature.
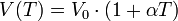
V0 = volume of gas at a temperature of zero degrees Celsius
V (T) = volume to a temperature greater than zero:
Alpha = expansion coefficient of gas (volume increase immediately by a unit volume gas when its temperature rises 1 ° C).
If the temperature is not taken as a reference in degrees centigrade but in degrees Kelvin, the law becomes:
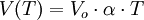
second law Gay Lussac : constant volume the pressure of a gas increases with temperature.

P0 = pressure of a gas at 0 ° C;
P (T) = pressure at a temperature greater than 0 ° C;
Alpha coefficient of expansion of the gas (relative increase of gas pressure when the temperature rises 1 ° C).
If the temperature is in degrees Kelvin, the law becomes:
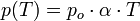
Both these laws are valid not too high pressures and temperatures close to the liquefaction of gases. It is therefore more occurred when a gas behaves like a perfect (ideal).
0 comments:
Post a Comment